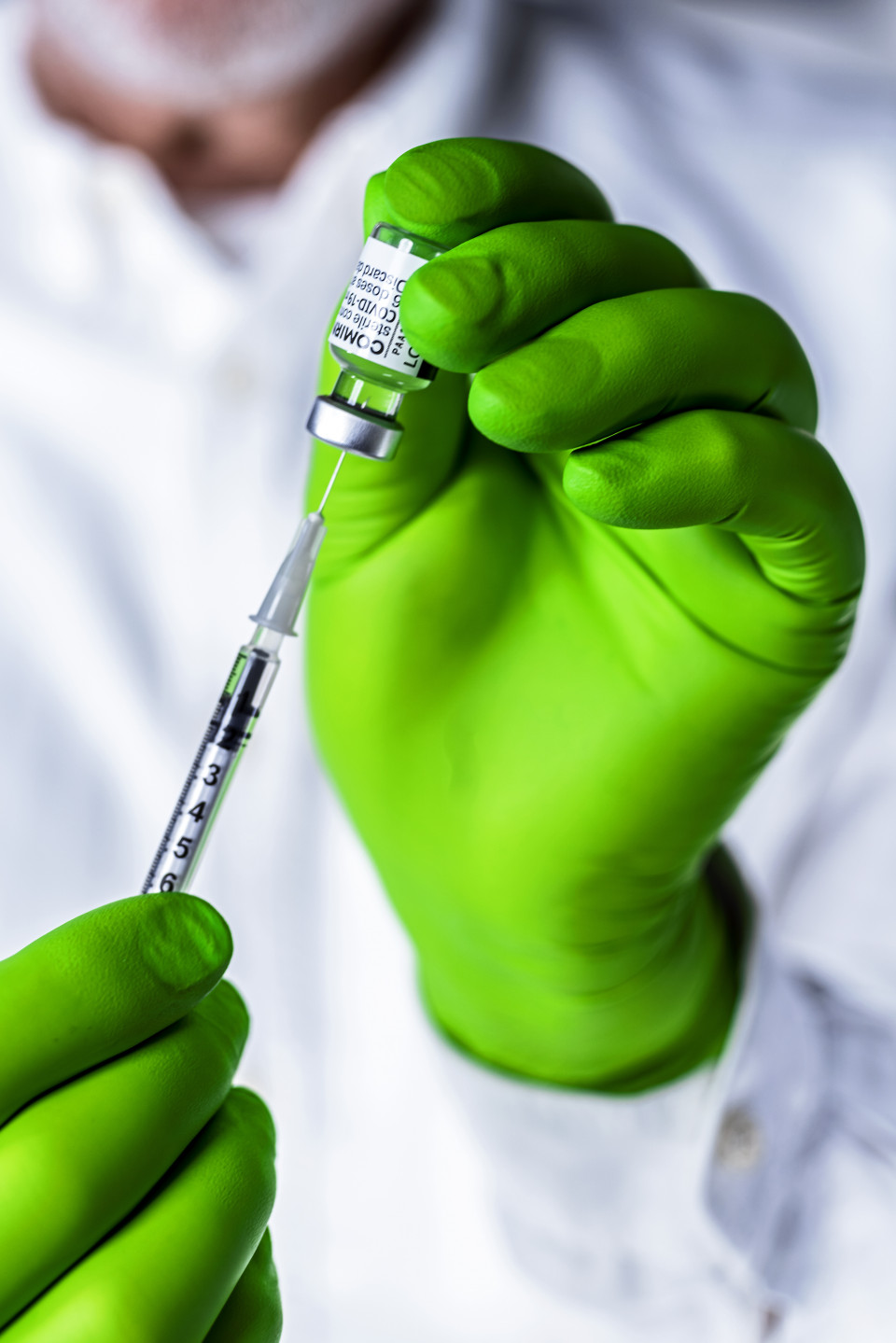
Winner 2021
mRNA vaccines for humanity

Prof. Dr. Uğur Şahin, Prof. Dr. Özlem Türeci and Prof. Dr. Christoph Huber as well as Prof. Dr. Katalin Karikó. have for several decades been working on the mRNA technology on which the COVID-19 vaccine from Pfizer/BioNTech is based. The nominees were the first to develop and get approval for a vaccine to prevent COVID-19 - in an unprecedented short period of time. They based the vaccine on the novel mRNA technology. This biomolecule, a ribonucleic acid, converts genetic information into proteins in cells, a process in medical speak known as encoding.
In developing a COVID-19 vaccine based on this technology, the team utilized their vast knowledge and decades of experience from their research into immunotherapies - specifically in the fight against cancer - based on mRNA. Uğur Şahin is Chief Executive Officer of BioNTech, Özlem Türeci is Chief Medical Officer. Christoph Huber is scientific advisor and a member of the Supervisory Board. He founded BioNTech in 2008 together with Uğur Şahin and Özlem Türeci. Katalin Karikó has worked for BioNTech since 2013 and has served as Senior Vice President since 2019.
More Details
Resume
Prof. Dr. med. Uğur Şahin (Sprecher)
- 1984 – 1990
- Medical School, University of Cologne
- 1987 – 1990
- Doctoral Thesis, Department of Internal Medicine, University of Cologne
- 1990 – 1991
- Internship, Department of Internal Medicine, University Cologne
- 1992
- PhD (Summa cum laude), University of Cologne
- 1992 – 1999
- Resident, Department of Internal Medicine, University Hospital of the Saarland (Homburg)
- 1999
- Post-doctoral Lecturer Qualification (“Habilitation”), Molecular Medicine & Immunology at the University of Homburg/Saar
- 2000 – 2001
- Visiting Scientist, Research group of Prof. Hans Hengartner and Prof. Rolf Zinkernagel, ETH and University Hospital, Zurich, Switzerland
- 2001 – 2016
- Co-founder and Chairman of the Clinical Advisory Board, Ganymed Pharmaceuticals GmbH (is now an Astellas Pharma subsidiary)
- 2001 – 2008
- Junior Research Group Leader, University Medical Center, Johannes Gutenberg University (Mainz)
- Since 2001
- Graduate Student Advisor, University Medical Center, Johannes Gutenberg University, Germany
- 2006 – 2013
- Associate Professor (W2), Experimental & Translational Oncology, University Medical Center, Johannes Gutenberg University (Mainz)
- Since 2009
- Co-founder and CEO, BioNTech
- 2010 – 2019
- Co-founder and Scientific Director, TRON – Translational Oncology at the University Medical Centre of the Johannes Gutenberg University gGmbH (Mainz)
- Since 2013
- Full Professor (W3), Translational Oncology & Immunology, University Medical Center, Johannes Gutenberg University (Mainz)
- Since 2018
- Chairman, Scientific Management Board, Helmholtz Institute for Translational Oncology (Mainz)
Other Activity and Honorary Posts
- Since 1992
- Referee, Nature, Science, Cell, Nature Medicine, Nature Biotechnology, Science Transl. Medicine, Nature Communications, Cancer Immunology Research, among others
- Since 2001
- Supervision of graduate students and postdoctoral fellows at the University Medical Center of Johannes Gutenberg University (Mainz)
- Since 2004
- Memberships of scientific societies, including American Society of Clinical Oncology, American Association for Cancer Research, Co-founder of CI3 Cluster of Individualized Immunointervention, Association for Cancer Immunotherapy, German Society for Immunology
- Since 2005
- Lecturer, Faculty of Medicine, Johannes Gutenberg University (Mainz)
- Since 2013
- Editorial Board Member, Cancer Immunology Research, American Association for Cancer Research
- Since 2014
- Organization of scientific meetings, including International “mRNA Health Conference” and “Keystone Symposium on Lymphocytes and their Roles in Cancer”
Patents
- Dr. Ugur Sahin is co-Inventor in more than 500 filed patents applications and patents.
Publications
- Liu Y, Liu J, Xia H, Zhang X, Zou J, Fontes-Garfias CR, Weaver SC, Swanson KA, Cai H, Sarkar R, Chen W, Cutler M, Cooper D, Muik A, Sahin U, Jansen KU, Xie X, Dormitzer PR, Shi PY. BNT162b2-Elicited Neutralization against New SARS-CoV-2 Spike Variants. N Engl J Med. 2021 Jul 29;385(5):472-474. doi: 10.1056/NEJMc2106083. Epub 2021 May 12.
Sahin, U., Muik, A., Vogler, I., Derhovanessian, E., Kranz, L. M., Vormehr, M., …., , Cooper, D., Kyratsous, C. A., Dormitzer, P. R., Jansen, K. U. & Türeci, Ö. BNT162b2 induces SARS-CoV-2-neutralising antibodies and T cells in humans. Nature (2021).
Krienke, C., Kolb, L., Diken, E., Streuber, M., Kirchhoff, S., Bukur, T., Akilli-Öztürk, Ö., Kranz, L. M., Berger, H., Petschenka, J., Diken, M., Kreiter, S., Yogev, N., Waisman, A., Karikó, K., Türeci, Ö. & Sahin, U. A noninflammatory mRNA vaccine for treatment of experimental autoimmune encephalomyelitis. Science 371, 145–153 (2021).
Vogel A, Kanevsky I, Che Y, Swanson KA, Muik A , Vormehr M , Kranz LM , Walzer K , Hein S, Güler A , Loschko J, Maddur M , Setlik A , Tompkins K , Cole J, .., Türeci Ö, Dormitzer PR, Jansen K , Sahin U. BNT162b vaccines protect non-human primates against SARS-CoV-2. Nature 2021.
Walsh, E. E., Frenck, R. W. J., Falsey, A. R., Kitchin, N., Absalon, J., Gurtman, A., Lockhart, S., Neuzil, K., Mulligan, M. J., Bailey, R., Swanson, K. A., Li, P., Koury, K., Kalina, W., Cooper, D., Fontes-Garfias, C., Shi, P.-Y., Türeci, Ö., Tompkins, K. R., Lyke, K. E., Raabe, V., Dormitzer, P. R., Jansen, K. U., Şahin, U. & Gruber, W. C. Safety and Immunogenicity of Two RNA-Based Covid-19 Vaccine Candidates. N. Engl. J. Med. (2020) doi:10.1056/NEJMoa2027906.
Beissert, T., Perkovic, M., Vogel, A., Erbar, S., Walzer, K. C., Hempel, T., Brill, S., Haefner, E., Becker, R., Türeci, Ö. & Sahin, U. A Trans-amplifying RNA Vaccine Strategy for Induction of Potent Protective Immunity. Mol. Ther. 28, 119–128 (2020).
Mulligan, M. J., Lyke, K. E., Kitchin, N., Absalon, J., Gurtman, A., Lockhart, S., Neuzil, K., Raabe, V., Bailey, R., Swanson, K. A., Li, P., Koury, K., Kalina, W., Cooper, D., Fontes-Garfias, C., Shi, P.-Y., Türeci, Ö., Tompkins, K. R., Walsh, E. E., Frenck, R., Falsey, A. R., Dormitzer, P. R., Gruber, W. C., Şahin, U. & Jansen, K. U. Phase I/II study of COVID-19 RNA vaccine BNT162b1 in adults. Nature 586, 589–593 (2020).
Sahin, U., Muik, A., Derhovanessian, E., Vogler, I., Kranz, L. M., Vormehr, M., Baum, A., Pascal, K., Quandt, J., Maurus, D., Brachtendorf, S., Lörks, V., Sikorski, J., Hilker, R., Becker, D., Eller, A.-K., Grützner, J., Boesler, C., Rosenbaum, C., Kühnle, M.-C., Luxemburger, U., Kemmer-Brück, A., Langer, D., Bexon, M., Bolte, S., Karikó, K., Palanche, T., Fischer, B., Schultz, A., Shi, P.-Y., Fontes-Garfias, C., Perez, J. L., Swanson, K. A., Loschko, J., Scully, I. L., Cutler, M., Kalina, W., Kyratsous, C. A., Cooper, D., Dormitzer, P. R., Jansen, K. U. & Türeci, Ö. COVID-19 vaccine BNT162b1 elicits human antibody and T(H)1 T cell responses. Nature 586, 594–599 (2020).
Reinhard, K., Rengstl, B., Oehm, P., Michel, K., Billmeier, A., Hayduk, N., Klein, O., Kuna, K., Ouchan, Y., Wöll, S., Christ, E., Weber, D., Suchan, M., Bukur, T., Birtel, M., Jahndel, V., Mroz, K., Hobohm, K., Kranz, L., Diken, M., Kühlcke, K., Türeci, Ö. & Sahin, U. An RNA vaccine drives expansion and efficacy of claudin-CAR-T cells against solid tumors. Science 367, 446–453 (2020).
Sahin, U., Oehm, P., Derhovanessian, E., Jabulowsky, R. A., Vormehr, M., Gold, M., Maurus, D., Schwarck-Kokarakis, D., Kuhn, A. N., Omokoko, T., Kranz, L. M., Diken, M., Kreiter, S., Haas, H., Attig, S., Rae, R., Cuk, K., Kemmer-Brück, A., Breitkreuz, A., Tolliver, C., Caspar, J., Quinkhardt, J., Hebich, L., Stein, M., Hohberger, A., Vogler, I., Liebig, I., Renken, S., Sikorski, J., Leierer, M., Müller, V., Mitzel-Rink, H., Miederer, M., Huber, C., Grabbe, S., Utikal, J., Pinter, A., Kaufmann, R., Hassel, J. C., Loquai, C. & Türeci, Ö. An RNA vaccine drives immunity in checkpoint-inhibitor-treated melanoma. Nature 585, 107–112 (2020).
Polack, F. P., Thomas, S. J., Kitchin, N., Absalon, J., Gurtman, A., Lockhart, S., Perez, J. L., Pérez Marc, G., Moreira, E. D., Zerbini, C., Bailey, R., Swanson, K. A., Roychoudhury, S., Koury, K., Li, P., Kalina, W. V, Cooper, D., Frenck, R. W. J., Hammitt, L. L., Türeci, Ö., Nell, H., Schaefer, A., Ünal, S., Tresnan, D. B., Mather, S., Dormitzer, P. R., Şahin, U., Jansen, K. U. & Gruber, W. C. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N. Engl. J. Med. 383, 2603–2615 (2020).
Orlandini von Niessen, A. G., Poleganov, M. A., Rechner, C., Plaschke, A., Kranz, L. M., Fesser, S., Diken, M., Löwer, M., Vallazza, B., Beissert, T., Bukur, V., Kuhn, A. N., Türeci, Ö. & Sahin, U. Improving mRNA-Based Therapeutic Gene Delivery by Expression-Augmenting 3’ UTRs Identified by Cellular Library Screening. Mol. Ther. 27, 824–836 (2019).
Sahin, U. & Türeci, Ö. Personalized vaccines for cancer immunotherapy. Science 359, 1355–1360 (2018).
Sahin, U., Derhovanessian, E., Miller, M., Kloke, B.-P., Simon, P., Löwer, M., Bukur, V., Tadmor, A. D., Luxemburger, U., Schrörs, B., Omokoko, T., Vormehr, M., Albrecht, C., Paruzynski, A., Kuhn, A. N., Buck, J., Heesch, S., Schreeb, K. H., Müller, F., Ortseifer, I., Vogler, I., Godehardt, E., Attig, S., Rae, R., Breitkreuz, A., Tolliver, C., Suchan, M., Martic, G., Hohberger, A., Sorn, P., Diekmann, J., Ciesla, J., Waksmann, O., Brück, A.-K., Witt, M., Zillgen, M., Rothermel, A., Kasemann, B., Langer, D., Bolte, S., Diken, M., Kreiter, S., Nemecek, R., Gebhardt, C., Grabbe, S., Höller, C., Utikal, J., Huber, C., Loquai, C. & Türeci, Ö. Personalized RNA mutanome vaccines mobilize poly-specific therapeutic immunity against cancer. Nature 547, 222–226 (2017).
Kranz, L. M., Diken, M., Haas, H., Kreiter, S., Loquai, C., Reuter, K. C., Meng, M., Fritz, D., Vascotto, F., Hefesha, H., Grunwitz, C., Vormehr, M., Hüsemann, Y., Selmi, A., Kuhn, A. N., Buck, J., Derhovanessian, E., Rae, R., Attig, S., Diekmann, J., Jabulowsky, R. A., Heesch, S., Hassel, J., Langguth, P., Grabbe, S., Huber, C., Türeci, Ö. & Sahin, U. Systemic RNA delivery to dendritic cells exploits antiviral defence for cancer immunotherapy. Nature 534, 396–401 (2016).
Kreiter, S., Vormehr, M., van de Roemer, N., Diken, M., Löwer, M., Diekmann, J., Boegel, S., Schrörs, B., Vascotto, F., Castle, J. C., Tadmor, A. D., Schoenberger, S. P., Huber, C., Türeci, Ö. & Sahin, U. Mutant MHC class II epitopes drive therapeutic immune responses to cancer. Nature 520, 692–696 (2015).
Sahin, U., Karikó, K. & Türeci, Ö. mRNA-based therapeutics--developing a new class of drugs. Nat. Rev. Drug Discov. 13, 759–780 (2014).
Kreiter, S., Diken, M., Selmi, A., Diekmann, J., Attig, S., Hüsemann, Y., Koslowski, M., Huber, C., Türeci, Ö. & Sahin, U. FLT3 ligand enhances the cancer therapeutic potency of naked RNA vaccines. Cancer Res. 71, 6132–6142 (2011).
Türeci, O., Koslowski, M., Helftenbein, G., Castle, J., Rohde, C., Dhaene, K., Seitz, G. & Sahin, U. Claudin-18 gene structure, regulation, and expression is evolutionary conserved in mammals. Gene 481, 83–92 (2011).
Diken, M., Kreiter, S., Selmi, A., Britten, C. M., Huber, C., Türeci, Ö. & Sahin, U. Selective uptake of naked vaccine RNA by dendritic cells is driven by macropinocytosis and abrogated upon DC maturation. Gene Ther. 18, 702–708 (2011).
Koslowski, M., Luxemburger, U., Türeci, O. & Sahin, U. Tumor-associated CpG demethylation augments hypoxia-induced effects by positive autoregulation of HIF-1α. Oncogene 30, 876–882 (2011).
Kuhn, A. N., Diken, M., Kreiter, S., Selmi, A., Kowalska, J., Jemielity, J., Darzynkiewicz, E., Huber, C., Türeci, O. & Sahin, U. Phosphorothioate cap analogs increase stability and translational efficiency of RNA vaccines in immature dendritic cells and induce superior immune responses in vivo. Gene Ther. 17, 961–971 (2010).
Kreiter, S., Selmi, A., Diken, M., Koslowski, M., Britten, C. M., Huber, C., Türeci, O. & Sahin, U. Intranodal vaccination with naked antigen-encoding RNA elicits potent prophylactic and therapeutic antitumoral immunity. Cancer Res. 70, 9031–9040 (2010).
Holtkamp, S., Kreiter, S., Selmi, A., Simon, P., Koslowski, M., Huber, C., Türeci, O. & Sahin, U. Modification of antigen-encoding RNA increases stability, translational efficacy, and T-cell stimulatory capacity of dendritic cells. Blood 108, 4009–4017 (2006).
Sahin, U., Türeci, O., Schmitt, H., Cochlovius, B., Johannes, T., Schmits, R., Stenner, F., Luo, G., Schobert, I. & Pfreundschuh, M. Human neoplasms elicit multiple specific immune responses in the autologous host. Proc. Natl. Acad. Sci. U. S. A. 92, 11810–11813 (1995).
Honors and Awards (Selection)
- 2012
- German Federal Ministry of Education and Research Peak Cluster (BMBF Spitzencluster) Award – member of CI3 Cluster for Individualized Immune Intervention
- 2018
- ERC Advanced Grant “SUMMIT” Improving Individualized Cancer Vaccines
- 2019
- Mustafa Prize
- 2019
- German Cancer Award
- 2021
- Axel Springer Award
- 2021
- Federal Cross of Merit
- 2021
- Hall of Fame German Science
- 2021
- Empress Theophano Prize
- 2021
- Precision Medicine World Conference (PMWC) Luminary Award
- 2021
- Election as a Member of European Molecular Biology Organization (EMBO)
- 2021
- Princess of Asturias Award in the category “Scientific Research”
PD Dr. med. Özlem Türeci
- 1986 – 1992
- Medical studies, Saarland University Faculty of Medicine (Homburg)
- 1989 – 1991
- Doctoral thesis, Institute of Human Genetics, Saarland University Faculty of Medicine (Homburg)
- 1992 – 1994
- Junior residency, 1st Department of Internal Medicine, Saarland University Medical Center (Homburg)
- 1993 – 1996
- Postdoctoral research, Saarland University Medical Center (Homburg)
- 1996 – 2000
- Research Group Leader, Saarland University Medical Center (Homburg)
- 2000 – 2010
- Research Group Leader, University Medical Center, Johannes Gutenberg University (Mainz)
- 2001 – 2004
- Scientific Technical Manager, European Cancer Immunome Program (EUCIP)
- 2001 - 2016
- Co-founder and CSO (2001 – 2008), CEO/CMO (2008 – 2016), Ganymed Pharmaceuticals AG (since 2016 an Astellas Pharma subsidiary)
- 2002
- Habilitation in “Molecular Medicine”, University Medical Center, Johannes Gutenberg University (Mainz)
- Since 2002
- Associate Professor/Lecturer (“Privatdozent”), University Medical Center, Johannes Gutenberg University (Mainz)
- 2003 – 2008
- Head of High-Density-Microarray Core Facility, University Medical Center, Johannes Gutenberg University (Mainz)
- 2009 – 2018
- Co-founder and Chair (Clinical and Scientific Advisory Board), BioNTech SE
- Since 2011
- Co-founder and Chair, Cluster of Individualized Immune Intervention Ci3 (Mainz)
- 2013 – 2016
- Postgraduate MSc studies, Regulatory Affairs for Biopharmaceutical Products program, EUCRAF/University of Strasbourg
- Since 2018
- Co-Founder and Chief Medical Officer, BioNTech SE
- Since 2019
- President, Association for Cancer Immunotherapy CIMT, Mainz
Other Activity and Honorary Posts
- 1992 – 2001
- Various lectures and seminars on immunology and biotechnology, Saarland University Faculty of Medicine (Homburg)
- 2004 – 2012
- Various lectures and seminars at the University Medical Center, Johannes Gutenberg University (Mainz)
Member in various scientific societies (incl. American Society of Clinical Oncology, American Association for Cancer Research, Association for Cancer Immunotherapy, Cluster of Individualized Immune Intervention, German Society for Immunology)
Commissions of trust/reviewer for various organizations (e.g. German Cancer Aid, Federal Ministry of Education and Research) and Journals (e.g. Journal of Immunology, Science)
Patents
- Dr. Özlem Türeci is co-Inventor in more than 500 filed international patent applications and patents.
Publikationen
- Sahin, U., Muik, A., Vogler, I., Derhovanessian, E., Kranz, L. M., Vormehr, M., …., , Cooper, D., Kyratsous, C. A., Dormitzer, P. R., Jansen, K. U. & Türeci, Ö. BNT162b2 induces SARS-CoV-2-neutralising antibodies and T cells in humans. Nature (2021).
Krienke, C., Kolb, L., Diken, E., Streuber, M., Kirchhoff, S., Bukur, T., Akilli-Öztürk, Ö., Kranz, L. M., Berger, H., Petschenka, J., Diken, M., Kreiter, S., Yogev, N., Waisman, A., Karikó, K., Türeci, Ö. & Sahin, U. A noninflammatory mRNA vaccine for treatment of experimental autoimmune encephalomyelitis. Science 371, 145–153 (2021).
Vogel A, Kanevsky I, Che Y, Swanson KA, Muik A , Vormehr M , Kranz LM , Walzer K , Hein S, Güler A , Loschko J, Maddur M , Setlik A , Tompkins K , Cole J, .., Türeci Ö, Dormitzer PR, Jansen K , Sahin U. BNT162b vaccines protect non-human primates against SARS-CoV-2. Nature 2021.
Alexander Muik, Ann-Kathrin Wallisch, Bianca Sänger, Kena A. Swanson, Julia Mühl, Wei Chen, Hui Cai, Ritu Sarkar, Özlem Türeci, Philip R. Dormitzer, U. S. Neutralization of SARS-CoV-2 lineage B.1.1.7 pseudovirus by BNT162b2 vaccine-elicited human sera. Science (2021).
Walsh, E. E., Frenck, R. W. J., Falsey, A. R., Kitchin, N., Absalon, J., Gurtman, A., Lockhart, S., Neuzil, K., Mulligan, M. J., Bailey, R., Swanson, K. A., Li, P., Koury, K., Kalina, W., Cooper, D., Fontes-Garfias, C., Shi, P.-Y., Türeci, Ö., Tompkins, K. R., Lyke, K. E., Raabe, V., Dormitzer, P. R., Jansen, K. U., Şahin, U. & Gruber, W. C. Safety and Immunogenicity of Two RNA-Based Covid-19 Vaccine Candidates. N. Engl. J. Med. (2020) doi:10.1056/NEJMoa2027906.
Beissert, T., Perkovic, M., Vogel, A., Erbar, S., Walzer, K. C., Hempel, T., Brill, S., Haefner, E., Becker, R., Türeci, Ö. & Sahin, U. A Trans-amplifying RNA Vaccine Strategy for Induction of Potent Protective Immunity. Mol. Ther. 28, 119–128 (2020).
Mulligan, M. J., Lyke, K. E., Kitchin, N., Absalon, J., Gurtman, A., Lockhart, S., Neuzil, K., Raabe, V., Bailey, R., Swanson, K. A., Li, P., Koury, K., Kalina, W., Cooper, D., Fontes-Garfias, C., Shi, P.-Y., Türeci, Ö., Tompkins, K. R., Walsh, E. E., Frenck, R., Falsey, A. R., Dormitzer, P. R., Gruber, W. C., Şahin, U. & Jansen, K. U. Phase I/II study of COVID-19 RNA vaccine BNT162b1 in adults. Nature 586, 589–593 (2020).
Sahin, U., Muik, A., Derhovanessian, E., Vogler, I., Kranz, L. M., Vormehr, M., Baum, A., Pascal, K., Quandt, J., Maurus, D., Brachtendorf, S., Lörks, V., Sikorski, J., Hilker, R., Becker, D., Eller, A.-K., Grützner, J., Boesler, C., Rosenbaum, C., Kühnle, M.-C., Luxemburger, U., Kemmer-Brück, A., Langer, D., Bexon, M., Bolte, S., Karikó, K., Palanche, T., Fischer, B., Schultz, A., Shi, P.-Y., Fontes-Garfias, C., Perez, J. L., Swanson, K. A., Loschko, J., Scully, I. L., Cutler, M., Kalina, W., Kyratsous, C. A., Cooper, D., Dormitzer, P. R., Jansen, K. U. & Türeci, Ö. COVID-19 vaccine BNT162b1 elicits human antibody and T(H)1 T cell responses. Nature 586, 594–599 (2020).
Reinhard, K., Rengstl, B., Oehm, P., Michel, K., Billmeier, A., Hayduk, N., Klein, O., Kuna, K., Ouchan, Y., Wöll, S., Christ, E., Weber, D., Suchan, M., Bukur, T., Birtel, M., Jahndel, V., Mroz, K., Hobohm, K., Kranz, L., Diken, M., Kühlcke, K., Türeci, Ö. & Sahin, U. An RNA vaccine drives expansion and efficacy of claudin-CAR-T cells against solid tumors. Science 367, 446–453 (2020).
Sahin, U., Oehm, P., Derhovanessian, E., Jabulowsky, R. A., Vormehr, M., Gold, M., Maurus, D., Schwarck-Kokarakis, D., Kuhn, A. N., Omokoko, T., Kranz, L. M., Diken, M., Kreiter, S., Haas, H., Attig, S., Rae, R., Cuk, K., Kemmer-Brück, A., Breitkreuz, A., Tolliver, C., Caspar, J., Quinkhardt, J., Hebich, L., Stein, M., Hohberger, A., Vogler, I., Liebig, I., Renken, S., Sikorski, J., Leierer, M., Müller, V., Mitzel-Rink, H., Miederer, M., Huber, C., Grabbe, S., Utikal, J., Pinter, A., Kaufmann, R., Hassel, J. C., Loquai, C. & Türeci, Ö. An RNA vaccine drives immunity in checkpoint-inhibitor-treated melanoma. Nature 585, 107–112 (2020).
Polack, F. P., Thomas, S. J., Kitchin, N., Absalon, J., Gurtman, A., Lockhart, S., Perez, J. L., Pérez Marc, G., Moreira, E. D., Zerbini, C., Bailey, R., Swanson, K. A., Roychoudhury, S., Koury, K., Li, P., Kalina, W. V, Cooper, D., Frenck, R. W. J., Hammitt, L. L., Türeci, Ö., Nell, H., Schaefer, A., Ünal, S., Tresnan, D. B., Mather, S., Dormitzer, P. R., Şahin, U., Jansen, K. U. & Gruber, W. C. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N. Engl. J. Med. 383, 2603–2615 (2020).
Orlandini von Niessen, A. G., Poleganov, M. A., Rechner, C., Plaschke, A., Kranz, L. M., Fesser, S., Diken, M., Löwer, M., Vallazza, B., Beissert, T., Bukur, V., Kuhn, A. N., Türeci, Ö. & Sahin, U. Improving mRNA-Based Therapeutic Gene Delivery by Expression-Augmenting 3’ UTRs Identified by Cellular Library Screening. Mol. Ther. 27, 824–836 (2019).
Sahin, U. & Türeci, Ö. Personalized vaccines for cancer immunotherapy. Science 359, 1355–1360 (2018).
Sahin, U., Derhovanessian, E., Miller, M., Kloke, B.-P., Simon, P., Löwer, M., Bukur, V., Tadmor, A. D., Luxemburger, U., Schrörs, B., Omokoko, T., Vormehr, M., Albrecht, C., Paruzynski, A., Kuhn, A. N., Buck, J., Heesch, S., Schreeb, K. H., Müller, F., Ortseifer, I., Vogler, I., Godehardt, E., Attig, S., Rae, R., Breitkreuz, A., Tolliver, C., Suchan, M., Martic, G., Hohberger, A., Sorn, P., Diekmann, J., Ciesla, J., Waksmann, O., Brück, A.-K., Witt, M., Zillgen, M., Rothermel, A., Kasemann, B., Langer, D., Bolte, S., Diken, M., Kreiter, S., Nemecek, R., Gebhardt, C., Grabbe, S., Höller, C., Utikal, J., Huber, C., Loquai, C. & Türeci, Ö. Personalized RNA mutanome vaccines mobilize poly-specific therapeutic immunity against cancer. Nature 547, 222–226 (2017).
Kranz, L. M., Diken, M., Haas, H., Kreiter, S., Loquai, C., Reuter, K. C., Meng, M., Fritz, D., Vascotto, F., Hefesha, H., Grunwitz, C., Vormehr, M., Hüsemann, Y., Selmi, A., Kuhn, A. N., Buck, J., Derhovanessian, E., Rae, R., Attig, S., Diekmann, J., Jabulowsky, R. A., Heesch, S., Hassel, J., Langguth, P., Grabbe, S., Huber, C., Türeci, Ö. & Sahin, U. Systemic RNA delivery to dendritic cells exploits antiviral defence for cancer immunotherapy. Nature 534, 396–401 (2016).
Kreiter, S., Vormehr, M., van de Roemer, N., Diken, M., Löwer, M., Diekmann, J., Boegel, S., Schrörs, B., Vascotto, F., Castle, J. C., Tadmor, A. D., Schoenberger, S. P., Huber, C., Türeci, Ö. & Sahin, U. Mutant MHC class II epitopes drive therapeutic immune responses to cancer. Nature 520, 692–696 (2015).
Sahin, U., Karikó, K. & Türeci, Ö. mRNA-based therapeutics--developing a new class of drugs. Nat. Rev. Drug Discov. 13, 759–780 (2014).
Kreiter, S., Diken, M., Selmi, A., Diekmann, J., Attig, S., Hüsemann, Y., Koslowski, M., Huber, C., Türeci, Ö. & Sahin, U. FLT3 ligand enhances the cancer therapeutic potency of naked RNA vaccines. Cancer Res. 71, 6132–6142 (2011).
Türeci, O., Koslowski, M., Helftenbein, G., Castle, J., Rohde, C., Dhaene, K., Seitz, G. & Sahin, U. Claudin-18 gene structure, regulation, and expression is evolutionary conserved in mammals. Gene 481, 83–92 (2011).
Diken, M., Kreiter, S., Selmi, A., Britten, C. M., Huber, C., Türeci, Ö. & Sahin, U. Selective uptake of naked vaccine RNA by dendritic cells is driven by macropinocytosis and abrogated upon DC maturation. Gene Ther. 18, 702–708 (2011).
Koslowski, M., Luxemburger, U., Türeci, O. & Sahin, U. Tumor-associated CpG demethylation augments hypoxia-induced effects by positive autoregulation of HIF-1α. Oncogene 30, 876–882 (2011).
Kuhn, A. N., Diken, M., Kreiter, S., Selmi, A., Kowalska, J., Jemielity, J., Darzynkiewicz, E., Huber, C., Türeci, O. & Sahin, U. Phosphorothioate cap analogs increase stability and translational efficiency of RNA vaccines in immature dendritic cells and induce superior immune responses in vivo. Gene Ther. 17, 961–971 (2010).
Kreiter, S., Selmi, A., Diken, M., Koslowski, M., Britten, C. M., Huber, C., Türeci, O. & Sahin, U. Intranodal vaccination with naked antigen-encoding RNA elicits potent prophylactic and therapeutic antitumoral immunity. Cancer Res. 70, 9031–9040 (2010).
Holtkamp, S., Kreiter, S., Selmi, A., Simon, P., Koslowski, M., Huber, C., Türeci, O. & Sahin, U. Modification of antigen-encoding RNA increases stability, translational efficacy, and T-cell stimulatory capacity of dendritic cells. Blood 108, 4009–4017 (2006).
Sahin, U., Türeci, O., Schmitt, H., Cochlovius, B., Johannes, T., Schmits, R., Stenner, F., Luo, G., Schobert, I. & Pfreundschuh, M. Human neoplasms elicit multiple specific immune responses in the autologous host. Proc. Natl. Acad. Sci. U. S. A. 92, 11810–11813 (1995).
Lectures
- Dr. Özlem Türeci has given more than 80 lectures at international and national scientific conferences and symposia (e.g. Science Asia, AACR, CICON, ESMO IO).
Honors and Awards (Selection)
- 2004
- Heisenberg Scholarship, German Research Foundation (DFG)
- 2005
- Georges Köhler Award, German Society for Immunology (DGFI)
- 2020
- German Sustainability Award
- 2021
- Axel Springer Award
- 2021
- Federal Cross of Merit
- 2021
- Hall of Fame German Science
- 2021
- Empress Theophano Prize
- 2021
- Precision Medicine World Conference Luminary Award
- 2021
- Election as a Member of European Molecular Biology Organization (EMBO)
- 2021
- Princess of Asturias Award in the category “Scientific Research”
Prof. Dr. med. Christoph Huber
- 1962 – 1968
- Medical studies and PhD, University of Innsbruck, Austria
- 1968 – 1974
- Specialist training for internal medicine, University of Innsbruck
- 1974 – 1975
- Research stays, e.g. at the Wallenberg Laboratory, Uppsala University, Sweden
- 1976
- Post-doctoral lecturer qualification (“Habilitation”) for internal medicine, focus on hematology-oncology and applied immunology, University of Innsbruck
- 1981
- Training in clinical bone marrow transplantation, Hutchinson Cancer Research Center, Seattle, USA
- 1983
- Founder and Head, Bone Marrow Transplantation Unit, University Hospital for Internal Medicine, Innsbruck
- 1986 – 2009
- Professor of Clinical Immunobiology and Head of the Department, University of Innsbruck
- 1990 – 2009
- Full Professor of Internal Medicine and Head of the Medical Clinic and Polyclinic III, University Medicine of the Johannes Gutenberg University, Mainz
- 2001 – 2016
- Co-Founder, Supervisory Board and scientific advisor, Ganymed Pharmaceuticals AG, Mainz
- Since 2008
- Co-Founder, Supervisory Board and scientific advisor, BioNTech SE, Mainz
- Since 2009
- Co-founder and scientific advisor, TRON – Translational Oncology at the University Medical Center of the Johannes Gutenberg University Mainz; now advisor to the Management and Supervisory Board
Other Activities and Honorary Posts
- 1990 – 1998
- Chairman of the Tumor Center Rhineland-Palatinate
- 1996 – 2008
- Speaker of the Collaborative Research Center 432 “Mechanisms of Tumor Defense and their Therapeutic Influence”
- 1997 – 2008
- Chairman of the Clinical Committee of the University Hospital Mainz
- Since 1997
- Member of the Austrian Academy of Sciences
- 2002 – 2019
- Founding President, since 2019 board member of the Association for Cancer Immunotherapy (CIMT)
- 2005 – 2009
- Chairman, Immunology Centre of Excellence Rhineland-Palatinate
- 2007 – 2011
- Chairman, commission for somatic gene therapy of the German Medical Council
- Since 2012
- Chairman, Cluster of Individualized Immune Intervention Ci3 (Mainz)
- Since 2019
- Founding president, European Network Cancer Immunotherapy (ENCI)
Prominent roles on research funding bodies of the DFG, the EU, German Cancer Aid, Cancer Research UK, the Swiss National Science Foundation and other organizations.
Honors and Awards (Selection)
- 2004
- Rhineland-Palatinate State Medal of Merit
- 2007
- Honorary Doctor, Medical University of Innsbruck
- 2015
- Honorary Medal of the Province of Tyrol
- 2015
- Federal Cross of Merit of the Federal Republic of Germany
- 2020
- Austrian Cross of Merit for Science and Art 1st Class
- 2021
- Honorary Doctor of the University Medicine Mainz
- 2021
- Honorary Member of the Austrian Academy of Sciences
Prof. Katalin Karikó, Ph.D.
- 1973 – 1978
- Study of Biology, University of Szeged (Hungary)
- 1978 - 1982
- PhD in Biochemistry, University of Szeged (Hungary)
- 1982 – 1985
- Postdoctoral fellow, Biological Research Center, Hungarian Academy of Sciences (Szeged, Hungary)
- 1985 – 1988
- Postdoctoral fellow, Department of Biochemistry, Temple University (Philadelphia, USA)
- 1988 – 1989
- Postdoctoral fellow, Department of Pathology, USUHS (Bethesda, USA)
- 1989 – 1995
- Research Assistant Professor, Department of Medicine, University of Pennsylvania (Philadelphia, USA)
- 1995 – 2009
- Senior Research Investigator, Department of Neurosurgery, University of Pennsylvania (Philadelphia, USA)
- 2006 – 2013
- Founder and CEO of RNARx, a company established to develop nucleoside-modified mRNA for therapy
- 2009 – 2021
- Adjunct Associate Professor, Department of Neurosurgery, University of Pennsylvania (Philadelphia, USA)
- 2013 – 2019
- Vice President, BioNTech
- Since 2019
- Senior Vice President, BioNTech
- Since 2021
- Adjunct Professor, Department of Neurosurgery, University of Pennsylvania (Philadelphia, USA)
Other Activity and Honorary Posts (Selection)
- Since 1992
- Member of the AAAS (American Association for the Advancement of Science)
- Since 2020
- Elected member of Academia Europaea
- Since 2013
- Organization of scientific meetings, including International “mRNA Health Conference” and “Keystone Symposium on Progress in Vaccine Development for Infectious Diseases”
- Since 2019
- Guest editor for Molecular Therapy 2019 (special issue on mRNA Therapy), for MDPI 2021 (Special Issue on mRNA Therapeutics: A Themed Issue in Honor of Professor Katalin Karikó)
Patents
- She is co-inventor in more 14 awarded international patents.
Publikationen
- Selected from 92 publications as they are the most relevant for mRNA drug development
Karikó, K., Kuo, A., Barnathan, E. S., and Langer, D. J: Phosphate-enhanced transfection of cationic lipid-complexed mRNA and plasmid DNA. Biochimica et biophysica acta 1369, 320, 1998.
Karikó, K., Kuo, A., and Barnathan, E: Overexpression of urokinase receptor in mammalian cells following administration of the in vitro transcribed encoding mRNA. Gene Ther 6, 1092, 1999.
Weissman, D., Ni, H., Scales, D., Dude, A., Capodici, J., McGibney, K., Abdool, A., Isaacs, S. N., Cannon, G., and Karikó, K: HIV gag mRNA transfection of dendritic cells (DC) delivers encoded antigen to MHC class I and II molecules, causes DC maturation, and induces a potent human in vitro primary immune response. J Immunol 165, 4710, 2000.
Karikó, K., Ni, H., Capodici, J., Lamphier, M., and Weissman, D: mRNA is an endogenous ligand for Toll-like receptor 3. J Biol Chem 279, 12542, 2004.
Koski, G. K., Karikó, K., Xu, S., Weissman, D., Cohen, P. A., and Czerniecki, B. J: Cutting Edge: Innate immune system discriminates between RNA containing bacterial versus eukaryotic structural features that prime for high-level IL-12 secretion by dendritic cells. J Immunol 172, 3989, 2004.
Karikó, K., Buckstein, M., Ni, H., and Weissman, D: Suppression of RNA recognition by Toll-like receptors: the impact of nucleoside modification and the evolutionary origin of RNA. Immunity 23, 165, 2005.
Karikó, K., Muramatsu, H., Welsh, F. A., Ludwig, J., Kato, H., Akira, S., and Weissman, D: Incorporation of pseudouridine into mRNA yields superior nonimmunogenic vector with increased translational capacity and biological stability. Mol Ther 16, 1833, 2008.
Anderson, B. R., Muramatsu, H., Nallagatla, S. R., Bevilacqua, P. C., Sansing, L. H., Weissman, D., and Karikó, K: Incorporation of pseudouridine into mRNA enhances translation by diminishing PKR activation. Nucleic acids research 38, 5884, 2010.
Karikó, K., Muramatsu, H., Ludwig, J., and Weissman, D: Generating the optimal mRNA for therapy: HPLC purification eliminates immune activation and improves translation of nucleoside-modified, protein-encoding mRNA. Nucleic acids research 39, e142, 2011.
Anderson, B. R., Muramatsu, H., Jha, B. K., Silverman, R. H., Weissman, D., and Karikó, K: Nucleoside modifications in RNA limit activation of 2'-5'-oligoadenylate synthetase and increase resistance to cleavage by RNase L. Nucleic acids research 39, 9329, 2011.
Karikó, K., Muramatsu, H., Keller, J. M., and Weissman, D: Increased erythropoiesis in mice injected with submicrogram quantities of pseudouridine-containing mRNA encoding erythropoietin. Mol Ther 20, 948, 2012.
Pardi, N., Tuyishime, S., Muramatsu, H., Karikó, K., Mui, B. L., Tam, Y. K., Madden, T. D., Hope, M. J., and Weissman, D: Expression kinetics of nucleoside-modified mRNA delivered in lipid nanoparticles to mice by various routes. J Control Release 217, 345, 2015.
Sahin, U., Karikó, K., and Türeci, Ö: mRNA-based therapeutics--developing a new class of drugs. Nat Rev Drug Discov 13, 759, 2014.
Stadler, C. R., Bahr-Mahmud, H., Celik, L., Hebich, B., Roth, A. S., Roth, R. P., Karikó, K., Tureci, Ö., and Sahin, U: Elimination of large tumors in mice by mRNA-encoded bispecific antibodies. Nature Medicine 23, 815, 2017.
Pardi, N., Hogan, M. J., Pelc, R. S., Muramatsu, H., Andersen, H., DeMaso, C. R., Dowd, K. A., Sutherland, L. L., Scearce, R. M., Parks, R., Wagner, W., Granados, A., Greenhouse, J., Walker, M., Willis, E., Yu, J. S., McGee, C. E., Sempowski, G. D., Mui, B. L., Tam, Y. K., Huang, Y. J., Vanlandingham, D., Holmes, V. M., Balachandran, H., Sahu, S., Lifton, M., Higgs, S., Hensley, S. E., Madden, T. D., Hope, M. J., Karikó, K., Santra, S., Graham, B. S., Lewis, M. G., Pierson, T. C., Haynes, B. F., and Weissman, D: Zika virus protection by a single low-dose nucleoside-modified mRNA vaccination. Nature 543, 248, 2017.
Pardi, N., Secreto, A. J., Shan, X., Debonera, F., Glover, J., Yi, Y., Muramatsu, H., Ni, H., Mui, B. L., Tam, Y. K., Shaheen, F., Collman, R. G., Karikó, K., Danet-Desnoyers, G. A., Madden, T. D., Hope, M. J., and Weissman, D: Administration of nucleoside-modified mRNA encoding broadly neutralizing antibody protects humanized mice from HIV-1 challenge. Nat Commun 8, 14630, 2017.
Baiersdörfer, M., Boros, G., Muramatsu, H., Mahiny, A., Vlatkovic, I., Sahin, U., and Karikó, K: A Facile Method for the Removal of dsRNA Contaminant from In Vitro-Transcribed mRNA. Mol Ther Nucleic Acids 15, 26, 2019.
Sahin, U., Muik, A., Derhovanessian, E., Vogler, I., Kranz, L. M., Vormehr, M., Baum, A., Pascal, K., Quandt, J., Maurus, D., Brachtendorf, S., Lorks, V., Sikorski, J., Hilker, R., Becker, D., Eller, A. K., Grutzner, J., Boesler, C., Rosenbaum, C., Kuhnle, M. C., Luxemburger, U., Kemmer-Bruck, A., Langer, D., Bexon, M., Bolte, S., Karikó, K., Palanche, T., Fischer, B., Schultz, A., Shi, P. Y., Fontes-Garfias, C., Perez, J. L., Swanson, K. A., Loschko, J., Scully, I. L., Cutler, M., Kalina, W., Kyratsous, C. A., Cooper, D., Dormitzer, P. R., Jansen, K. U., and Tureci, O: COVID-19 vaccine BNT162b1 elicits human antibody and TH1 T cell responses. Nature 586, 594, 2020.
Sahin, U., Muik, A., Vogler, I., Derhovanessian, E., Kranz, L. M., Vormehr, M., Quandt, J., Bidmon, N., Ulges, A., Baum, A., Pascal, K., Maurus, D., Brachtendorf, S., Lörks, V., Sikorski, J., Koch, P., Hilker, R., Becker, D., Eller, A.-K., Grützner, J., Tonigold, M., Boesler, C., Rosenbaum, C., Heesen, L., Kühnle, M.-C., Poran, A., Dong, J. Z., Luxemburger, U., Kemmer-Brück, A., Langer, D., Bexon, M., Bolte, S., Palanche, T., Schultz, A., Baumann, S., Mahiny, A. J., Boros, G., Reinholz, J., Szabó, G. T., Karikó, K., Shi, P.-Y., Fontes-Garfias, C., Perez, J. L., Cutler, M., Cooper, D., Kyratsous, C. A., Dormitzer, P. R., Jansen, K. U., and Türeci, Ö: BNT162b2 induces SARS-CoV-2-neutralising antibodies and T cells in humans. Nature 595, 572, 2021.
Krienke, C., Kolb, L., Diken, E., Streuber, M., Kirchhoff, S., Bukur, T., Akilli-Öztürk, Ö., Kranz, L. M., Berger, H., Petschenka, J., Diken, M., Kreiter, S., Yogev, N., Waisman, A., Karikó, K., Türeci, Ö., and Sahin, U: A noninflammatory mRNA vaccine for treatment of experimental autoimmune encephalomyelitis. Science 371, 145, 2021
Lectures by invitation (last 5 years)
- Katalin Karikó has given more than 30 lectures by invitation at international and national scientific conferences and symposia (e.g., mRNA Health Conference, Cold Spring Harbor, RNA Medicine Symposium).
- 2016
- In vitro-transcribed mRNA for therapy and vaccination – Imperial College, London, UK
New frontiers: mRNA therapy – possible implications in burn wound treatment – 26th Conference of the European Wound Management Association, Bremen, Germany
Optimal mRNA in the making: RNA in clinical trials – 4th mRNA Health Conference, Boston, USA
Messenger RNA for therapy – RNA-Medicine: From RNA Discoveries to Future Therapies Berlin, Germany - 2017
- mRNA-based protein replacement therapies – Cluster for Individualized Immune Intervention (Ci3), Mainz, Germany
Developing mRNA therapy for protein replacement – RNA & Oligonucleotide Therapeutics, Cold Spring Harbor Laboratory, NY, US
From bench to bedside: In vitro transcribed mRNA for therapy – University of Debrecen, Hungary
In vitro transcribed mRNA for therapy – SFNano Summer School: Nucleic acids-based strategies to control gene expression, La Grande Motte, France
In vitro transcribed mRNA for therapy – Transgene, Illrich France
In vitro transcribed mRNA for therapy – Symposium of Medical RNomics University of Giessen, Giessen, Germany - 2018
- mRNA therapy – Icahn School of Medicine at Mount Sinai, NYC, USA
mRNA-based protein replacement therapy – Masters Seminar Series, University of Giessen, Germany
Modified mRNA platform – Pfizer, Pearl River, NY, USA - 2020
- My story of the mRNA – mRNA Day TriLink, San Diego, CA, USA
- 2021
- Development mRNA for therapy – UC Davis, Carrol C. Cross Distinguished Professorship lecture series, virtual presentation
Development mRNA for therapy – Brandeis University, USA, virtual presentation
Development mRNA for therapy – Pfizer, North America Medical Affairs, virtual
Az mRNS fejlesztése terápiára – Hungarian Academy of Sciences, opening the 194th year of the Academy, Budapest, Hungary
Development mRNA for therapy – 6th Annual RNA Medicine 2021Symposium, Beth Israel Deaconess Medical Center, virtual presentation
Developing mRNA for Therapy – Harvard Medical School, virtual symposium
Immune activation by RNA – Keystone Symposia, DAMPs Across the Tree of Life Inducing Innate Immunity, virtual presentation Az mRNS fejlesztése terápiára – Magyar Higiénikusok Társasága, Fodor József emlékülés
Development of the mRNA Therapeutic - Not Warp Speed! – Rosalind Franklin Society, Women in Science lecture, virtual presentation
Honors and Awards
- 2021
- Rosenstiel Award for Distinguished Work in Medical Science, Brandeis University
- 2021
- Reichstein medal, Swiss Academy of Pharmaceutical Sciences
- 2021
- Jedlik Ányos Award, Hungarian Intellectual Property Office
- 2021
- Fodor József Award, Hungarian Society of Hygiene
- 2021
- Princess of Asturias Award, Princess of Asturias Foundation, Spain
- 2021
- Széchenyi Prize, Hungarian Government
- 2021
- Semmelweis Award, Hungarian Government
- 2021
- Public Health Award, Research!America’s Outstanding Achievement
- 2021
- Wilhelm Exner Medal, Austrian Trade Association
- 2021
- Honorary Doctoral Degree, University of Szeged, Hungary
- 2022
- Vilcek Prize for Excellence in Biotechnology, Vilcek Foundation
Contact
Press
Julia Bloes
Manager Corporate Communications
BioNTech SE
An der Goldgrube 12
55131 Mainz
Phone: +49 (0) 6131 / 90 840
E-Mail: media@biontech.de
Web: www.biontech.de
A description provided by the institutes and companies regarding their nominated projects
In December 2020, BioNTech received the world’s first emergency or conditional market authorizations for its COVID-19 vaccine, the world’s first mRNA product. By developing the first mRNA drug, the company made medical history. BioNTech’s decades of research and development of immunotherapies are now making a difference in a way that the company always wanted to: for people around the world.
Prof. Dr. Ugur Sahin, Prof. Dr. Özlem Türeci and Dr. Christoph Huber founded BioNTech in 2008 as an immunotherapy company with the vision of ushering in a new era of immunotherapies and developing innovative approaches against cancer and infectious diseases. They were supported by Dr. Thomas and Dr. Andreas Strüngmann, Michael Motschmann and Helmut Jeggle. Dr. Katalin Karikó joined BioNTech in 2013 as Senior Vice President. In their work as practicing physicians, Dr. Sahin and Dr. Türeci have witnessed that innovations from science and research too rarely reach cancer patients. Then and now are they pursuing the goal of using their research findings to develop solutions that improve the health of people worldwide by harnessing the full potential of the immune system.
With its founding, BioNTech began developing individualized as well as off-the-shelf cancer therapies. 2020 was a transformative year for BioNTech. In 2020, in what the company called “Project Lightspeed”, BioNTech developed a well-tolerated and effective vaccine for all mankind to address the COVID-19 pandemic. In doing so, the team demonstrated not only the potential of mRNA technology, but also the success of decades of research.
Project Lightspeed: The Development of the COVID-19 Vaccine
When the coronavirus spread in early 2020, there were some indications that it could beocme a global pandemic. BioNTech felt it had a duty to leverage its research expertise and make an important contribution to addressing the emerging global health crisis. BioNTech contributed the technology and expertise in immunology, while its partners Pfizer and Fosun Pharma drew on their established infrastructure, working with BioNTech to successfully conduct clinical trials and establish manufacturing and distribution.
A breakthrough has truly been achieved at the speed of light. In less than a year, BioNTech and its partner Pfizer developed a vaccine – a process that usually takes five to ten years. This was by far the fastest vaccine development in the history of medicine – without taking any shortcuts.
The vaccine is now being supplied to more than 100 countries. By end of July, 2021, more than 1 billion doses of the COVID-19 vaccine have already been delivered. For 2021 BioNTech, together with its partners Pfizer and Fosun, has so far signed orders for more than 2 billion doses. In addition, 2 billion doses of vaccine will be provided to low- and middle-income countries. For low-income countries, the vaccine will be offered at a “not-for-profit” price.
The company achieved numerous milestones on the road to approval of the COVID-19 vaccine. This was made possible by the following key factors:
Rapid development: BioNTech was the first company to bring an mRNA-based vaccine into clinical development in Europe. The vaccine was also the world’s first COVID-19 vaccine to receive conditional and emergency authorizations following the successful completion of the Phase 3 trial. BioNTech did not take any shortcuts during development, testing and regulatory approval, but successfully worked with all partners and regulatory agencies to reduce wait times and minimize idle time.
High efficacy and safety: Data from studies of vaccinations in Israel show that the vaccine not only offers more than 97 percent protection against symptomatic disease, severe disease and deaths, but also 94 percent protection against infection with SARS-CoV-2.
Quick adaptability: Studies of the variants observed to date demonstrate that the vaccine also protects effectively against newly emerging variants of the virus. Moreover, should an adaptation of the vaccine nevertheless become necessary, the mRNA vaccine platform enables a quick and flexible adaptation of the vaccine within six weeks.
Scaled-up production capacity: To achieve the goal of making the vaccine available worldwide, BioNTech scaled up production capacity in 2020 as early as possible and as quickly as possible. To this end, the company, at its own risk, acquired a production facility from Novartis in Marburg early on and expanded its global manufacturing and supplier network to include more than a dozen partners. This will enable the company to produce up to one billion doses of COVID-19 vaccine in 2021 through BioNTech’s own production network alone. For 2021, BioNTech expects a total production capacity of up to three billion doses.
Outlook: Harnessing the potential of the human immune system
Based on the knowledge gained from the vaccine program, research can be advanced even faster and more broadly in the future. After all, the global pandemic effort has set new standards for the development of vaccines and therapeutics.
BioNTech intends to build on this in future research. The clear goal is to bring further novel therapies in the areas of oncology and infectious diseases to market maturity within the next five years. In the long term, BioNTech also sees applications for technologies in allergies, inflammatory diseases, and even regenerative medicine. BioNTech established research programs in these areas early on and plans to expand them further in the coming years.
As immune engineers, BioNTech’s scientists and researchers continue to consistently pursue the vision of improving the health of people around the world by harnessing the full potential of the immune system.
About BioNTech
Biopharmaceutical New Technologies is a next-generation immunotherapy company pioneering the development of therapies for cancer and other serious diseases. The company combines a variety of advanced therapeutic platforms and bioinformatics tools to rapidly advance the development of novel biopharmaceuticals. Its diversified portfolio of oncology product candidates includes individualized therapies as well as off-the-shelf mRNA-based drugs, innovative chimeric antigen receptor (CAR) T cells, bispecific checkpoint immunomodulators, targeted cancer antibodies and small molecules. Based on its extensive expertise in mRNA vaccine development and in-house manufacturing capabilities, BioNTech is developing several mRNA vaccine candidates for a range of infectious diseases with collaboration partners, in addition to its diverse oncology pipeline. BioNTech works side-by-side with world-renowned pharmaceutical industry collaborators including Genmab, Sanofi, Bayer Animal Health, Genentech (a Roche company), Regeneron, Genevant, Fosun Pharma and Pfizer.
For more information, please visit: www.BioNTech.de.

 Gebärdensprache
Gebärdensprache
 Leichte Sprache
Leichte Sprache