Nominee 2009
Email-Botschaft vom Herzen

Under the direction of Dr. Hans-Jürgen Wildau BIOTRONIK has developed a technology that is unique worldwide for this purpose that uses automatic communication between a pacemaker or implanted defibrillator (ICD) and a data center. As Vice President Health Services, Dr. Wildau is responsible for research, development, operations and new business development in the area of telemonitoring and sensor systems at the medical device company BIOTRONIK in Berlin.
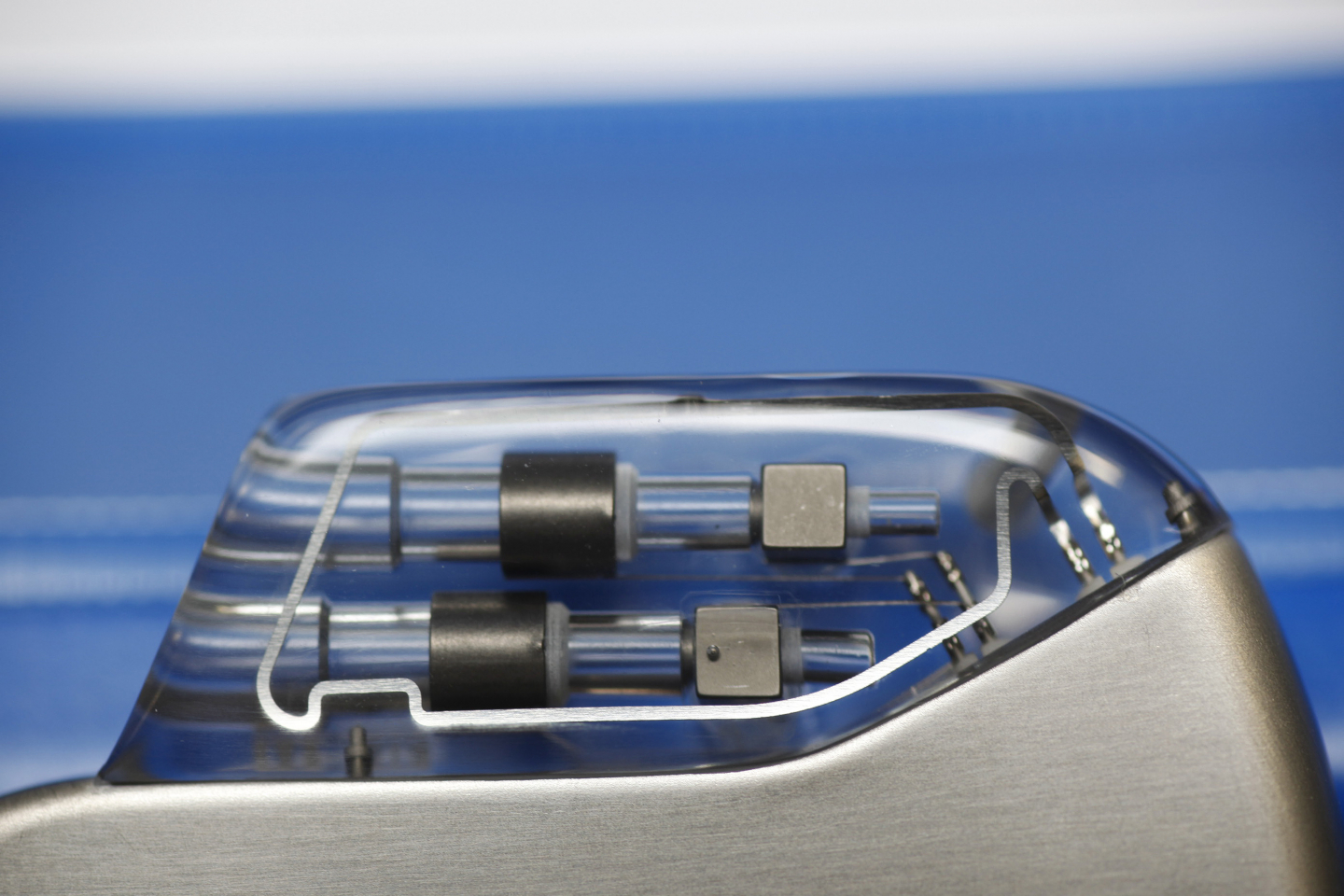
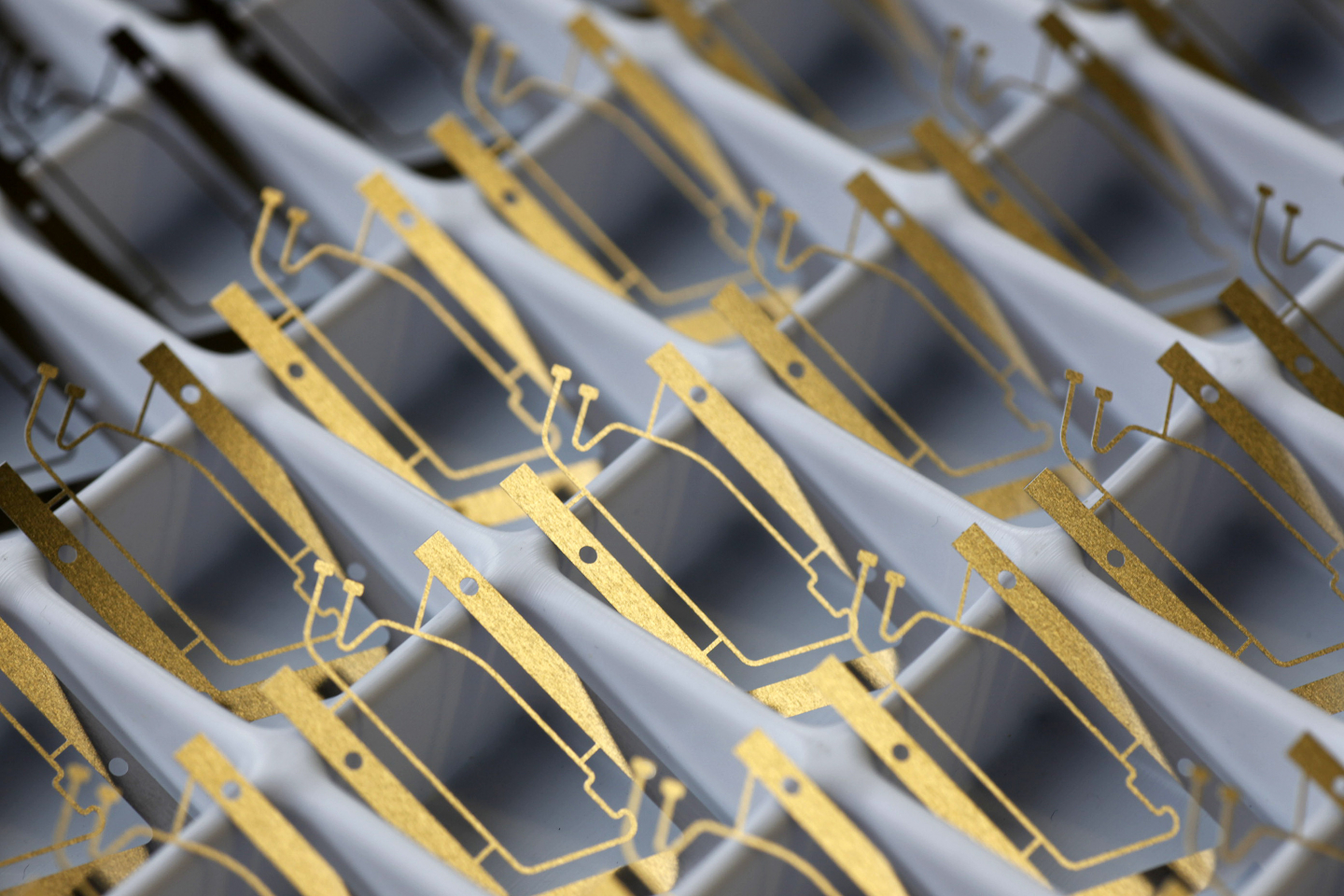
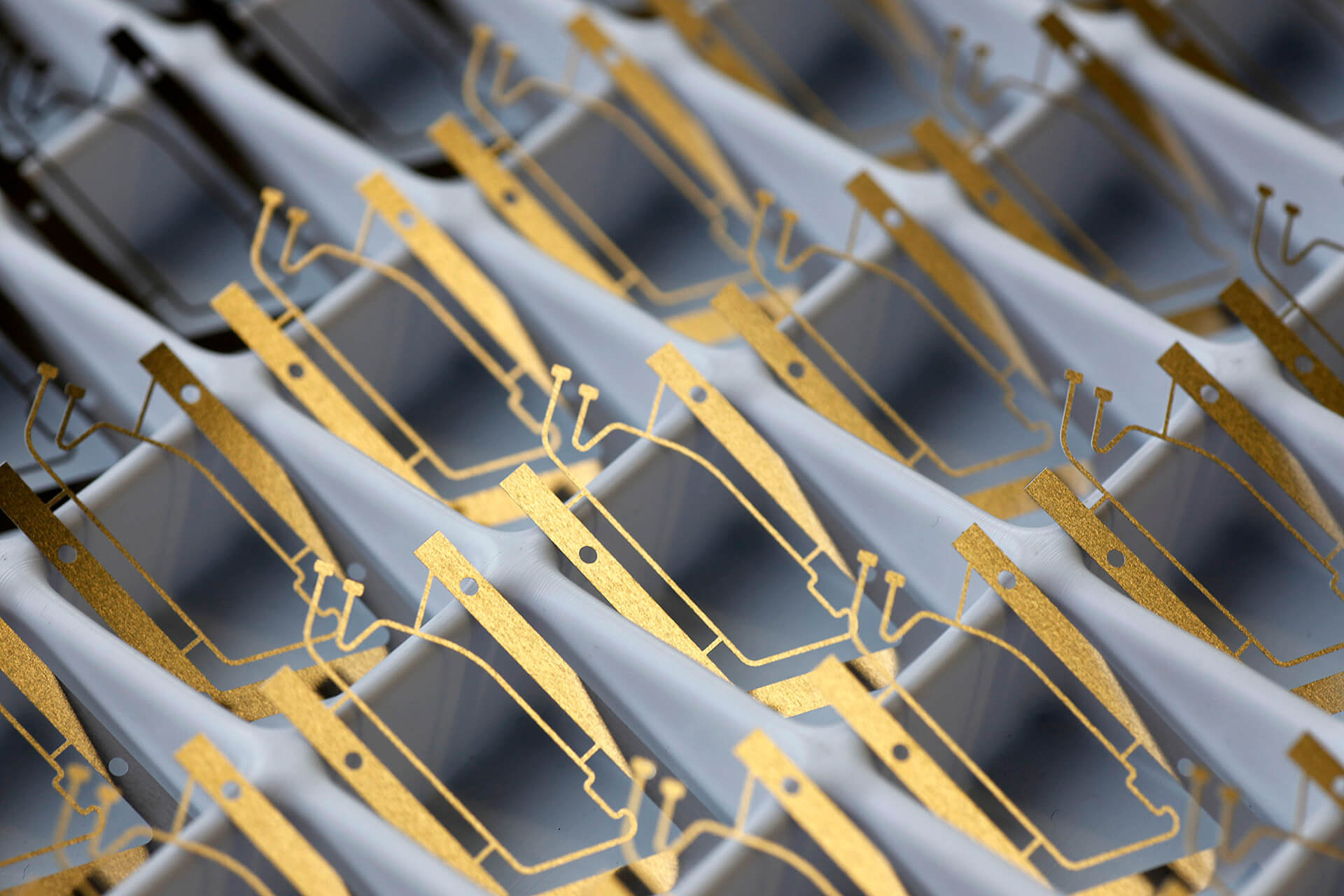
A main element of the communicative Home Monitoring system is an antenna in the head of a pacemaker or ICD. It is so small that it can be easily integrated in the implant. Its power consumption is minimal – the transmitter module only uses around one-hundredth of the battery capacity.

more details
Resumes

Dr.-Ing. Hans-Jürgen Wildau
- 07.10.1964
- geboren in Berlin
- 1982
- Abitur
- 1983 – 1989
- Studium der Elektrotechnik, Technischen Universität Berlin, Berlin
- 1987
- Dreimonatiges Forschungspraktikum, IBM, Böblingen
- 1989
- Diplom an der Technischen Universität Berlin
- 1989 – 1993
- Wissenschaftlicher Mitarbeiter, Fachbereich Elektrotechnik an der Technischen Universität Berlin
- 1992 – 1995
- Berufsbegleitendes Studium der Betriebswirtschaftslehre an der Fernuniversität Hagen
- 1993
- Promotion, Institut für Werkstoffe der Elektrotechnik, Technische Universität Berlin, Thema aus dem Gebiet Mikroelektronik und Halbleitertechnologie
- 1993 – 1997
- Produkt Manager, Marketing und Produktmanagement in der Medizintechnikindustrie, BIOTRONIK GmbH & Co. KG, Berlin
- 1997 – 1999
- Strategieberatung bei Roland Berger & Partner, München. Schwerpunkte: Medizintechnik- und Mikroelektronikindustrie
- 1999
- Wiederaufnahme der Tätigkeit, Geschäftsbereichsleiter, BIOTRONIK GmbH & Co. KG, Berlin
- seit 2000
- Vice President Health Services, BIOTRONIK SE & Co. KG, Berlin verantwortlich für Forschung, Entwicklung, Operations und neue Geschäftsentwicklung für Telemonitoring und Sensorik
Weitere Tätigkeiten:
- seit 2005
- Vorsitz des Beirates der BMBF-Initiative „Präventive Mikromedizin mit 24/7-Monitoring“
- seit 2009
- Aufnahme in das Kuratorium des Fraunhofer Instituts für Mikroelektronische Schaltungen und Systeme (IMS)
- 2009
- World Congress 2009 Medical Physics and Biomedical Engineering, Track Chair „Active Implants“
Mitgliedschaften:
- Verein Deutscher Ingenieure (VDI)
Deutsche Gesellschaft für Kardiologie (DGK)
Arbeitsgruppe Telemonitoring der DGK
Contact
Spokesperson
Dr.-Ing. Hans-Jürgen Wildau
Vice President Health Services
BIOTRONIK SE Co. KG
Woermannkehre 1
12359 Berlin
Tel.: +49 (0) 30 / 68 90 52 000
Fax: +49 (0) 30 / 68 90 52 940
E-Mail: hans-juergen.wildau@biotronik.com
Web: www.biotronik.de
Press
Amela Malja
Director Marketing Communications
BIOTRONIK SE Co. KG
Woermannkehre 1
12359 Berlin
Tel.: +49 (0) 30 / 68 90 51 400
Fax: +49 (0) 30 / 68 90 51 940
E-Mail: amela.malja@biotronik.com
Web: www.biotronik.de
A description provided by the institutes and companies regarding their nominated projects
Automatic remote monitoring of patients with arrhythmia
Cellular phones and the internet have changed all of our lives – yet for the chronically ill they have a very special potential. Home monitoring puts these technologies in the service of cardiology: through automatic, wireless remote monitoring physicians can keep track of their patients at all times, even when they have long since left the hospital. Patients with cardiac irregularities who wear a pacemaker or implantable defibrillator (ICD) thus receive much better medical care, experience more assurance and a better quality of life.
A pacemaker with Home Monitoring transmits medical and technical data daily. With this seamless information, the attending physician can monitor the course of treatment. He sees whether the implant is reacting optimally to cardiac signals and whether the selected course of drug treatment is having the desired effect. Health risks can be detected as they arise and thus much earlier than is possible under conventional care – and he can ask the patient to schedule an in-office follow-up to adjust treatment.
The system functions worldwide without patient intervention
As a pioneer in the field of telecardiology, BIOTRONIK developed this promising form of diagnosis very early and made it a commercial success. In the process, the company has relied on decades of experience in the research and production of pacemakers and ICDs. Internet-based Home Monitoring was designed in such a way that the patient does not have to initiate transmission himself. Automatic data transmission is thus a significant technical feature – and an important prerequisite for the safe application of the technology in medical care. The technology is already in use in 55 countries, around 200,000 devices with BIOTRONIK Home Monitoring® have already been implanted, more than 3,500 clinics are using remote monitoring.
Antenna, mobile patient devices and the internet: the information chain
The active implants (pacemakers, ICDs, cardiac resynchronization therapy systems) transmit cardiac as well as technical data via cellular phone networks. An antenna in the implant and a mobile patient device make this possible.
The antenna was integrated in the so-called header of the implant, the physiologically shaped “head” that also receives the leads. It transmits all important information generally once per day to CardioMessenger®, a cordless external patient device.
CardioMessenger® can e.g. be placed on a nightstand or clipped to a belt. It transmits relevant data via the T-Mobile global cellular phone network to a service center. The IBM technology computer center with BIOTRONIK software automatically decodes, analyzes, and stores transmitted data.
Via a protected internet connection, the attending physician can monitor the care of his patients anywhere in the world. Naturally, the interpretation of all data is reserved for the physician. The secured website, however, consolidates and clearly documents long-term trends as well as unique cardiac events or changes, such as e.g. a change in cardiac output. Patients requiring intervention or program adjustment soon are noted on the website. The physician also receives an event alert via email, SMS or fax in the event of a clinically relevant change in status.
Home Monitoring should not be confused with an emergency or rescue system. Health-related changes and changes relevant to further treatment are monitored, but uninterrupted data transfer via cellular networks cannot be guaranteed. If problems arise and in emergency situations, the patient must call for a physician via the known emergency numbers.
Full surgery hours, latent risks: Consideration of the outcome and aim of the innovation
In aging societies requiring a high degree of medical care, life with a pacemaker or ICD is becoming a reality for more and more people. More than one million patients worldwide currently receive a pacemaker, an ICD or a CRT system every year. Active implants control heart rate by means of electrical signals. In this way, they balance out prolonged and often life-threatening arrhythmias. A patient’s quality of life is considerably improved, even if the cause of the arrhythmia persists.
Clinicians generally only receive information on the status of patients during in-office or hospital visits – e.g. if their condition has deteriorated noticeably or a regular routine examination (2-4x per year) is scheduled. Only then are stored data read from the implant, only then can the cardiologist identify changes in an ECG, deterioration of cardiological status, health risks, or technical defects.
In conventional care, a secondary condition, such as auricular fibrillation, can develop without obvious symptoms in the time between in-office visits – and the cardiologist may not notice until weeks or months later. With automatic, continuous remote monitoring, however, the physician would receive a corresponding message and be able to react to the risk in an appropriate time period.
Studies look at advantages for patients, physicians, and healthcare
The innovation consequently pursued several targets: remote monitoring should improve the quality of treatment, provide the patient with greater assurance and mobility, and also ensure cost-efficient care.
Several clinical trials document the advantages achieved to date for physicians and patients. For example, the study proved that critical cardiac events were discovered 2 to 5 months earlier with Home Monitoring than with conventional in-office follow-up. Undesirable complications and side-effects were detected and the safety of patients improved (A. Lazarus, AWARE study, PACE 2007, 30: S2–S12.).
Another study revealed that remote monitoring with Home Monitoring can actually replace in-office visits – while providing patients with the same level of safety and care. According to the TRUST study, the number of in-office follow-ups for patients with defibrillators (ICD) was able to be reduced by more than 43%. Symptomatic and asymptomatic arrhythmias were detected faster with Home Monitoring and treated (N. Varma et al., TRUST trial, Circulation 2008, 118, 2309-2317, Abstract 4078).
Remote monitoring with Home Monitoring can thus not only save many older patients unnecessary trips to and waits in doctors’ offices – they also cut healthcare costs. While time and costs are saved in many routine cases, resources can be focused on those patients requiring immediate diagnosis, consultation and treatment.
Remote interrogation as the patient sleeps: What makes BIOTRONIK Home Monitoring® unique
BIOTRONIK has nine years of clinical experience behind it supported by trials with the technology. In market comparisons, BIOTRONIK Home Monitoring® is the only system with fully automatic, mobile data transmission. No patient intervention is required: data can be transmitted while the patient sleeps. Thanks to the automatic mode of operation, potential operating errors and information gaps are eliminated.
The following facts are of interest as technical innovations: the radio technology was integrated without changing the implant size and with high energy efficiency. The Home Monitoring function only uses around 1% of the capacity of a pacemaker battery – despite daily use over a period of many years. The company has also developed CardioMessenger®, a mobile patient device for worldwide mobile communication transmission based on GSM. The device is designed to be highly reliable. With the exception of the activation function, there is nothing for the patient to do; handling errors are thus as good as impossible.
The BIOTRONIK software for data analyses is capable of detecting deviations from standard values reliably. This is the only way digital medicine can be developed manageably because the personal and detailed collating of all transmitted data would be so much more than the physician and his resources could handle. For this purpose, a corresponding internet platform has been made available. The physician uses the platform to view data analyzed by the software. A traffic light system codes patient reports by urgency and thereby helps the physician to use time and work efficiently. All required technologies – implant, cellular system, internet – have been integrated in a global approved medical technology system available around the clock.
About BIOTRONIK GmbH & Co. KG
As one of the world’s leading cardiovascular medical device companies with several million implanted devices, BIOTRONIK is represented in over 100 countries with 4,500 employees. The company was founded more than 50 years ago by Prof. Dr. Max Schaldach, the inventor of the first German pacemaker, in Berlin. Company headquarters is still in the German capital city, and company headquarters still employ more than 1,600 employees in the divisions Development, Production, Marketing, Sales, and Administration.
The right to nominate outstanding achievements for the Deutscher Zukunftspreis is incumbent on leading German institutions in science and industry as well as foundations.
The project “Messages of the heart – pacemakers send emails to physicians” was nominated by the Federal Ministry of Education and Research.


 Gebärdensprache
Gebärdensprache
 Leichte Sprache
Leichte Sprache